Xom Cau Vong

Xom Cau Vong

Xom Cau Vong
HIV Tests
More than 300k+ people have used community-based HIV testing & self-testing
PrEP users
More than 81k+ people have enrolled in PrEP
News channel for the gay community and LGBTQ-related social activities in Vietna
Visit now!
News channel for transgender women and LGBTQ-related social activities in Vietnam
Visit now!
Feature News
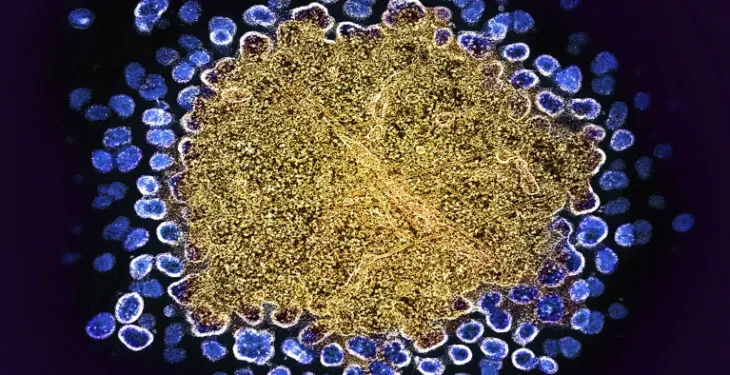
Latest HIV Cure Case Comes With a Twist
(Medscape) The first HIV cure case in which the stem cell donor had a single — rather than double — gene mutation is opening the donor pool in renewed cure efforts to make stem cell transplants more widely available.
The anonymous patient is the first to achieve long-term HIV remission — in this case approaching 6 years — after receiving a single CCR5 delta 32 mutation. Specifically, the patient received a CCR5 wild-type, delta 32 transplant, known as a heterozygous transplant, for acute myeloid leukemia, investigators reported at the International AIDS Conference 2024 in Munich, Germany.
Other cures have involved donors with two copies of the CCR5 delta 32 mutation, known as homozygous.
Researchers are calling the anonymous patient “the next Berlin patient,” an homage to Timothy Ray Brown, the first, now renowned, American patient living at the time in Germany who was cured of HIV.
Brown — dubbed an ambassador of hope — would never test positive for HIV again, but at age 54, it would be the leukemia that led to his HIV cure that would take Brown’s life after spreading to his brain and spinal cord.
Expanding the Donor PoolSince then, four others who received the dual-copy mutation in a homozygous stem cell transplant have experienced long-term HIV remission.
Another case, known as the “Geneva patient,” received a stem cell transplant from a wild-type CCR5 donor. Researchers from Geneva, Switzerland, reported that case last year at the conference.
Using donors with single mutations in addition to those with double mutations could meaningfully expand the pool of donors and the availability of allogeneic stem cell transplantation, said Christian Gaebler, MD, coleader of the personalized infectious medicine program at the Berlin Institute of Health and associate professor at Charité – Universitätsmedizin Berlin, who presented the case at the conference.
“When we don’t find a donor with these delta 32 mutations — and it’s hard to find them, especially in geographical regions outside western or northern countries where it’s almost impossible to find a homozygous delta 32 donor — it may be beneficial to take a heterozygous donor,” Gaebler said during an interview. “They’re easier to find.”
“This case is giving us hope that there is still a cure and underlying mechanisms that we’re currently not understanding,” said Christoph Spinner, MD, MBA, an infectious disease specialist at the University Hospital of the Technical University of Munich, and AIDS 2024 conference co-chair.
“Research is needed to understand and translate the findings of this case for the cure research around the globe,” he said.
Reducing the HIV ReservoirThe key mechanism in a cure is depletion of the HIV reservoir, Gaebler said, but more work is needed to better understand its role.
The most recent cured patient had the stem cell transplant to treat acute myeloid leukemia initially, Gaebler explained, and more than 5 years after the stem cell transplant and after discontinuing antiretroviral therapy, the patient has undetectable levels of HIV DNA and HIV RNA as well as higher levels of CD4+ and CD8+ T cells, he said.
“When we see this next Berlin patient and that we’re coming close to 6 years of HIV remission, I think we can quite confidently say we can have HIV reservoir reduction, HIV remission, and potentially HIV cure independent of the CCR5 status,” Gaebler said.
“These initial cases of HIV cure have triggered a lot of studies looking at CCR5 and basically modulating CCR5 expression, gene-edited cells, [and] CCR5 blockage,” he pointed out after his presentation. “This is all very valid and it will likely play a role, but it probably comes down to a combination of these things,” he said.
Source: Medscape
…Seventh patient ‘cured’ of HIV: why scientists are excited
A man in Germany is HIV-free after receiving stem cells that are not resistant to the virus.
A 60-year-old man in Germany has become at least the seventh person with HIV to be announced free of the virus after receiving a stem-cell transplant1. But the man, who has been virus-free for close to six years, is only the second person to receive stem cells that are not resistant to the virus.
“I am quite surprised that it worked,” says Ravindra Gupta, a microbiologist at the University of Cambridge, UK, who led a team that treated one of the other people who is now free of HIV2,3. “It’s a big deal.”
The first person found to be HIV-free after a bone-marrow transplant to treat blood cancer4 was Timothy Ray Brown, who is known as the Berlin patient. Brown and a handful of others received special donor stem cells2,3. These carried a mutation in the gene that encodes a receptor called CCR5, which is used by most HIV virus strains to enter immune cells. To many scientists, these cases suggested that CCR5 was the best target for an HIV cure.
The latest case — presented at the 25th International AIDS Conference in Munich, Germany, this week — turns that on its head. The patient, referred to as the next Berlin patient, received stem cells from a donor who only had one copy of the mutated gene, which means their cells do express CCR5, but at lower levels than usual.
The case sends a clear message that finding a cure for HIV is “not all about CCR5”, says infectious-disease physician Sharon Lewin, who heads The Peter Doherty Institute for Infection and Immunity in Melbourne, Australia.
Ultimately, the findings widen the donor pool for stem-cell transplants, a risky procedure offered to people with leukaemia but unlikely to be rolled out for most individuals with HIV. Roughly 1% of people of European descent carry mutations in both copies of the CCR5 gene, but some 10% of people with such ancestry have one mutated copy5.
The case “broadens the horizon of what might be possible” for treating HIV, says Sara Weibel, a physician-scientist who studies HIV at the University of California, San Diego. Some 40 million people are living with HIV globally.
Six years HIV-freeThe next Berlin patient was diagnosed with HIV in 2009. He developed a type of blood and bone-marrow cancer known as acute myeloid leukaemia in 2015. His doctors could not find a matching stem-cell donor who had mutations in both copies of the CCR5 gene. But they found a female donor who had one mutated copy, similar to the patient. The next Berlin patient received the stem-cell transplant in 2015.
“The cancer treatment went very well,” says Christian Gaebler, a physician-scientist and immunologist at the Charité — Berlin University Medicine, who presented the work. Within a month, the patient’s bone-marrow stem cells had been replaced with the donor’s. The patient stopped taking antiretroviral drugs, which suppress HIV, in 2018. And now, almost six years later, researchers can’t find evidence of HIV replicating in the patient.
Shrunken reservoirPrevious attempts to transplant stem cells from donors with regular CCR5 genes have seen the virus reappear weeks to months after the people with HIV stopped taking antiretroviral therapy, in all but one person6. In 2023, Asier Sáez-Cirión, an HIV researcher at the Pasteur Institute in Paris, presented data on an individual called the Geneva patient, who had been without antiretroviral therapy for 18 months7. Sáez-Cirión says the person remains free of the virus, about 32 months later.
Researchers are now trying to work out why these two transplants succeeded when others have failed.
They propose several mechanisms. First, antiretroviral treatment causes the amount of virus in the body to drop considerably. And chemotherapy before the stem-cell transplant kills many of the host’s immune cells, which is where residual HIV lurks. Transplanted donor cells might then mark leftover host cells as foreign and destroy them, together with any virus residing in them. The rapid and complete replacement of the host’s bone-marrow stem cells with those of the donor’s might also contribute to the swift eradication. “If you can shrink the reservoir enough, you can cure people,” says Lewin.
The fact that both the next Berlin patient and his stem cell donor had one CCR5 gene copy with a mutation could have created an extra barrier to the virus entering cells, says Gaebler.
The case also has implications for therapies currently in early-stage clinical trials, in which the CCR5 receptor is sliced out of a person’s own cells using CRISPR–Cas9 and other gene-editing techniques, says Lewin. Even if these therapies don’t get to every single cell, they could still have an impact, she says.
Source: Nature
…With mpox cases on the rise, advocates urge vaccinations to avoid potential resurgence
More than two years since the initial mpox outbreak, cases are trending up again — and the word still hasn’t gotten out to everyone at risk. During Pride Month, public health officials came armed with a serious message: Get vaccinated.
“Since mpox is not really in the news right now, they don’t necessarily feel a need,” Army Cachero told CBS News.
Cachero and the public health outreach team at the University of California, Los Angeles are concerned about a resurgence of mpox, which broke out across the U.S. and other countries in 2022. Only 23% of those at risk nationwide are vaccinated.
The symptoms of the virus include severe rash and blistering, body aches and fever — and it can be deadly.
Jose Velasquez didn’t need his arm twisted to roll up his sleeve. He remembers the fear and pain of being infected with mpox in 2022.
“I was actually hospitalized. I got to the point that my lymph nodes were really swollen,” Velasquez said. With the shot, he says, “I feel a little relieved.”
Men who have sex with men and trans women are at highest risk. But there are barriers to getting the most vulnerable protected with the highly effective two-dose vaccine.
Cachero says misinformation, lack of health care and lack of trust all play a role in why vaccination rates are lower among people of color.
Across the country, public health officials are scrambling to prevent a repeat of the panic and confusion of the summer of 2022.
Nick Diamond and his husband, Keletso Makofane, are public health advocates in New York.
“I think it’s really personal because it’s our friends and our neighbors who are concerned about infection,” Diamond said.
Their research found that many people at risk connect online and hook up in private homes — and that public health messaging must adapt.
“That requires new thinking, that requires collaboration and that requires urgency and speed. If we don’t build that muscle, we are going to suffer more than we need to suffer,” Makofane said.
Source: CBS News
…Toihen.vn app
The Toihen.vn webpage and app helps people set up appointments for HIV counseling and testing, pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) counseling and treatment, sexually transmitted diseases counseling and treatment, and many other services. Living by the motto of "Peace of mind - Trust - Speed - Sharing”, Toi Hen will always help you connect with LGBTQ-friendly clinics and community-based organizations in the fastest and most secure way! Try Toi Hen today!
Explore nowPrEP app
Did you already start using PrEP? Then you know how important it is to follow the PrEP plan properly! Download the iPrEP application to get reminders to take PrEP medicine on time and have regular follow-up health checks. iPrEP will also provide you with important information about PrEP at each stage of your PrEP use.
Explore now